![\includegraphics[width=8cm]{bel1.eps}](img1.gif)
Fig.1. Schematic design of the equipment for thermocyclic treatment.
Changes of the electron concentration and mobility in CdTeHg semiconductor alloys subjected to repeated heating-cooling cycles were studied. The role of the initial crystal properties as well as of the glue material keeping the sample on the substrate in the observed changes was examined.
PACS numbers: 81.40.Rs, 72.20.Fr
1. Introduction
Measuring set-ups for semiconductor crystals as well as semiconductor devices themselves work sometimes under thermally unstable conditions. After repeated uses they can present wrong results of the measurements or the results which do not correspond to the actual conditions of the experiment.
Some of us observed such undesired effects while working in winter with such devices as photodetectors repeatedly outside and inside of a chamber with the ambient temperature correspondingly of 250 K and 300 K. Very often semiconductor devices change their properties after multiple temperature shocks.
The main aim of this work was to search for the optimum conditions of the semiconductor sample assembling to avoid the above-mentioned effects.
2. Experimental
We performed two series of experiments. In the first one we observed the changes of electrical properties of semiconductor samples subjected to repeated heating-cooling cycles. In the other one we investigated the influence of gluing the sample on the observed changes.
Semiconducting compound CdxHg1-xTe was chosen as a material for investigations because this alloy attracts the interest of solid state physicists owing to its applications in optoelectronics [1].
![\includegraphics[width=8cm]{bel1.eps}](img1.gif)
The construction of the measuring set-up is shown in Fig. 1. There is a container for liquid nitrogen (1) in which the sample while putting is cooled down to a temperature of 77 K. A heater (2) allows us to warm the sample up to a temperature of 350 K. The sample (3) is placed inside an aluminum capsule hanging up on a thread. The thread is strengthened on adjustable disc (5) next passes through a roller (4). A belt (6) joins the shaft of the disc with that of electric motor. While the motor works the sample alternately rises up to the heater and drops down to the liquid nitrogen. This way a very large number of thermocycles could be achieved in a limited time period.
The basic electrical characteristic of a semiconductor crystal, its
electrical conductivity, is dependent on free electron concentration, n,
and mobility, ![]() . Therefore we used the Hall-effect measurement which is
a standard method for determining concentration and mobility of free
current carriers in solids.
. Therefore we used the Hall-effect measurement which is
a standard method for determining concentration and mobility of free
current carriers in solids.
An electromagnet allowed to apply a magnetic field up to 5 kOe. In order to avoid an influence of magnetic hysteresis on the measuring accuracy the electromagnet was first brought to saturation and next a necessary value of the magnetic field, H, was settled. The compensation measuring scheme was used in which the voltage drop across the sample contacts and the intensity of the current flowing through the sample were measured by digital volt-ampere-ohmmeter.
The Hall voltage, ![]() , (which follows from the Lorentz force acting
on an electron moving perpendicularly to the magnetic field) was measured
on two pairs of contacts under two directions of both the magnetic fields
and the current, I, flowing through the sample. The Hall coefficient,
, (which follows from the Lorentz force acting
on an electron moving perpendicularly to the magnetic field) was measured
on two pairs of contacts under two directions of both the magnetic fields
and the current, I, flowing through the sample. The Hall coefficient,
![]() equals [2]
equals [2]
A voltage drop measured across two other pairs of contacts was used to
determine the specific resistance of the sample
![]() . Then we
could calculate the mobility of free electrons from the equation
. Then we
could calculate the mobility of free electrons from the equation
![]() [3].
[3].
3. Results
In the first part of the experiments we studied the variation of the
specific resistivity, ![]() , electron concentration, n, and electron
mobility,
, electron concentration, n, and electron
mobility, ![]() , as a function of the number of thermocycles. The
experimental data are presented in Figs. 2-3. We observe remarkable
changes in
, as a function of the number of thermocycles. The
experimental data are presented in Figs. 2-3. We observe remarkable
changes in ![]() and
and ![]() after a number of thermocycles. It is seen from
Figs. 2(a, b, c) that the changes of the measured parameters in samples of
the thickness
after a number of thermocycles. It is seen from
Figs. 2(a, b, c) that the changes of the measured parameters in samples of
the thickness ![]() mm start just after 150-200 thermocycles. It is
noteworthy that more essential alterations of
mm start just after 150-200 thermocycles. It is
noteworthy that more essential alterations of ![]() and
and ![]() occur in the
samples with lower electron mobility. Thus, if one has an opportunity to
select the samples before device fabrication, it would be better to take
the samples with higher mobility. The sample 3 can serve as an example in
our case. The mobility changes in this samples cannot come out from an
experimental error after 350 thermocycles. At the same time in the sample 1
a remarkable drop in the mobility occurs already after 150
thermocycles.
occur in the
samples with lower electron mobility. Thus, if one has an opportunity to
select the samples before device fabrication, it would be better to take
the samples with higher mobility. The sample 3 can serve as an example in
our case. The mobility changes in this samples cannot come out from an
experimental error after 350 thermocycles. At the same time in the sample 1
a remarkable drop in the mobility occurs already after 150
thermocycles.
![\includegraphics[width=15cm]{bel2.eps}](img15.gif)
Fig.2. Dependence of the electron mobility(a), the free electron concentration n (b), the specific resistivity
(c) on the number of thermocycles N for thick samples (
-0.1 mm), T=77 K.
In order to visualise the effect of the thermal treatment on the sample we applied the following procedure. The samples were inspected using a metallographic microscope (whose magnification was x 510, that was enough for our purpose). A large number of microcraks was observed on the sample surface after the thermocyclic treatment.
![\includegraphics[width=15cm]{bel3.eps}](img19.gif)
Fig.3. Dependence of the electron mobility(a), the free electron concentration n (b), the specific resistivity
(c) on the number of thermocycles N for thin films (
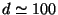
m), T=77 K.
An interesting effect was noticed while studying thin samples. The samples
of very similar initial parameters were found to display noticeably
different values of ![]() ,
, ![]() and n already after 50-100
thermocycles. To elucidate the reasons of this scatter we carried out
additional experiments paying attention to the influence of glue materials
used for keeping the sample. The results of these experiments are presented
in Figs. 3(a, b, c). We used the General Electric lacquer (sample 1) as
well as different types of epoxy cements (samples 2-4). It is seen from
Figs. 3(a, b, c) that the samples glued on a substrate using the epoxy
cements behave themselves stable during thermocyclic treatment. An opposite
behaviour takes place when a sample is glued on the substrate with the
General Electric lacquer. This can be explained by a difference in
thermo-mechanical characteristics of glue materials.
and n already after 50-100
thermocycles. To elucidate the reasons of this scatter we carried out
additional experiments paying attention to the influence of glue materials
used for keeping the sample. The results of these experiments are presented
in Figs. 3(a, b, c). We used the General Electric lacquer (sample 1) as
well as different types of epoxy cements (samples 2-4). It is seen from
Figs. 3(a, b, c) that the samples glued on a substrate using the epoxy
cements behave themselves stable during thermocyclic treatment. An opposite
behaviour takes place when a sample is glued on the substrate with the
General Electric lacquer. This can be explained by a difference in
thermo-mechanical characteristics of glue materials.
4. Discussion
Irreversible changes in the defect structure occur in the samples under thermocyclic treatment which are reflected by the changes of free electron concentration and mobility. Let us consider the possible reasons that could lead to the observed effects.
The change of temperature affects not only the atom diffusion and the defect structure in the investigated crystals, but may be also a reason for energy losses in the case of non-uniform temperature distribution throughout the sample cross section. On the one hand the sample volume is changing (extending or compressing) owing to the temperature alteration. On the other hand, according to the thermodynamic reciprocity principle, the volume deformation leads to a change of sample temperature. When the deformation is uniform, the resulting temperature distribution inside the sample is still uniform. In the opposite case, i.e. when deformation is non-uniform (for example, under bending some regions of the sample are extended and other ones are compressed), the temperature of the sample will also be non-uniform. As a result the temperature gradients through the sample cross section arise and thermal flows appear thus creating an additional inelastic deformation. If a strain which stimulates such a non-uniform deformation is changed periodically, the energy scattering takes place. This is, so-called, thermo-elastic effect described in detail by A.F. Ioffe [2].
At high frequency of the temperature variations an essential heat transfer
does not occur and the ratio between the strain and the deformation is
determined by the adiabatic elastic modulus. On the other hand, at low
frequency of the temperature variations the thermal equilibrium can take
place at many points of each cycle. The process of heat transfer is then
isothermic and it is characterised by isothermal elastic modulus. The rate
of this process depends on the sample size and its physical properties and
may by written in the following manner:
In which manner the above-mentioned process linked up with the samples under the study? It is observed that after a definite number of thermal cycles an inelastic deformation of samples occurs. This effect depends solely on the profile of temperature distribution. Thermal strains arise owing to a non-uniform distribution of temperature inside the sample volume while there are no thermal strains when temperature is constant. The original sample obviously comprises both point- and line-defects of crystal lattice which in turn can be sources of generation of new lattice defects. Such generation of defects can occur during every thermal cycle. Thus, the large is the number of initial lattice defects in a sample the larger is the number of newly generated defects per cycle.
Consequently, in the case of thick samples the intensive generation of defects takes place in the near-surface region where the magnitude of temperature gradients is sufficiently large. In the case of thin samples the changes of their properties are mainly determined by the difference in thermal coefficients of the glue and of the sample, but not on weak temperature gradients.
5. Conclusion
In conclusion it should be stated that efficiency of semiconductor devices exploited under temperature-unstable conditions depends, to some extend, on the properties of semiconductor crystal which is the active part of a device. To produce devices insensitive to thermal shocks it is recommended to take into account the following advices resulting from the present work:
1. to choose crystals with higher electron mobility;
2. to pay attention to the characteristics of glue used for keeping the crystal, such as their mechanical strengthening, thermal expansion coefficient, etc., and to the difference of those from the same parameters of the semiconductor crystal.
One more remark should be added. Usually devices are fabricated in the following manner: semiconductor crystal, being the heart of the device, is cut out from an ingot, then it undergoes mechanical and chemical treatment and thin wafer is glued on a substrate. In order to check the thermal stability of the device it is important to examine a thin layer mounted on the substrate, but not a bulk crystal. In my opinion, the right choice of testing methods is a principal way to improve the quality of devices.
Acknowledgments
Experimental part of this work was performed in the Institute of Semiconductor Physics, Academy of Sciences of Ukraine. I would like to thank Dr. S. Komirenko and Mrs. L.M. Kholvinskaya for their help and support during this work as well as for fruitful discussions and advices.