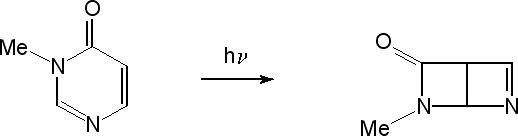
RESEARCH
METHOD
|
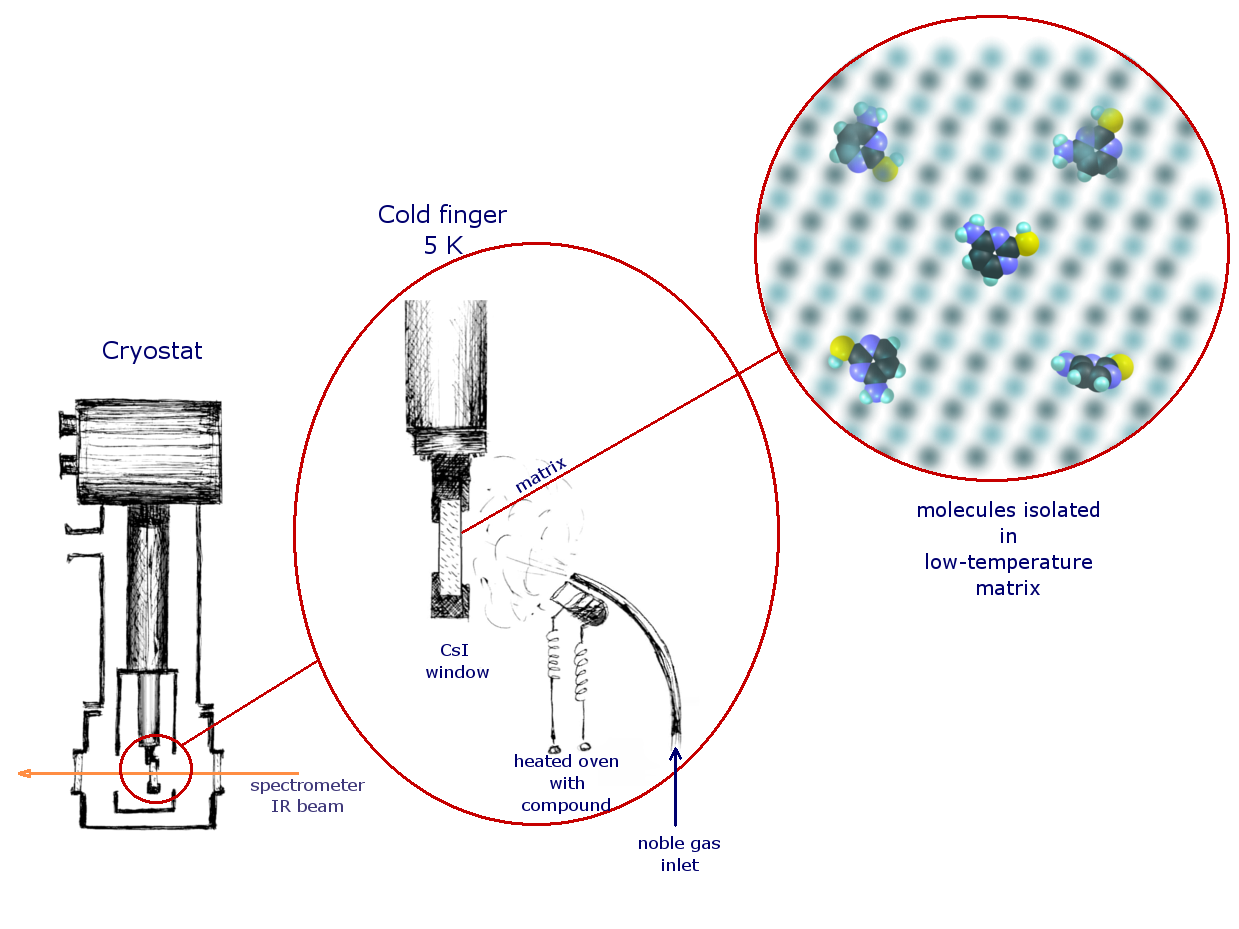
Scheme
of the experiment
 |
TOPICS
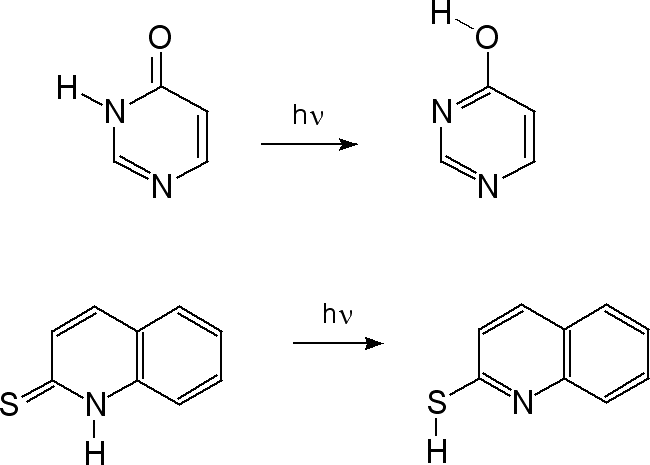
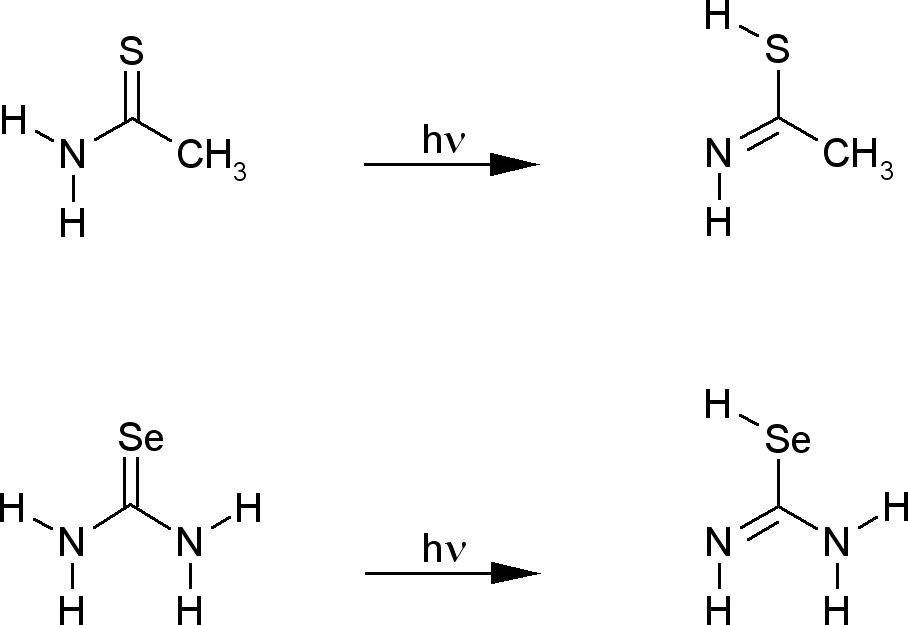
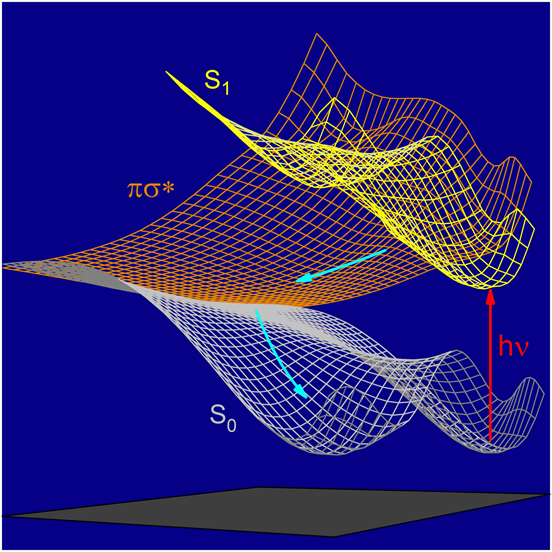
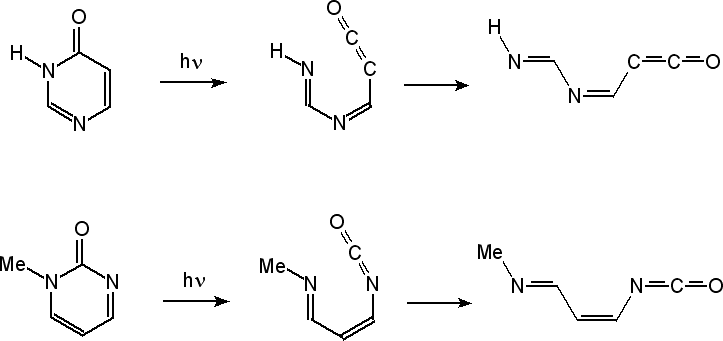
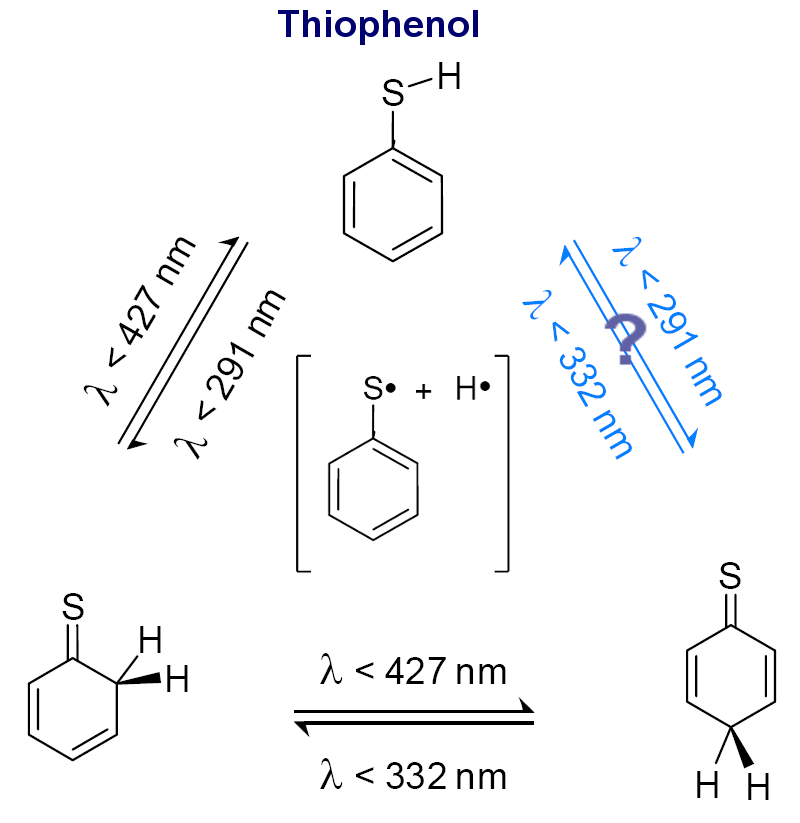
| Research of the group concentrates mainly on investigations of unimolecular photochemical transformations of nucleic acid bases, their derivatives and analogues. Photochemical transformations of monomeric molecules of these chemical species, isolated in low-temperature matrices, are induced by excitation with UV or NIR light of an appropriate wavelength. Two inherent features of matrix isolation: [i] very low temperature, 10 K or lower, [ii] inert chemical environment, precluding interactions with any potential reagents, make this technique a very useful tool for traping and stabilizing primary products of photochemical transformations. Because the matrix cage (usually) does not allow migrations of reagents and photogenerated species, the photoreactions observed using matrix-isolation method concern, in the most part, photoisomerizations.
Several
types of photoisomerization reactions were studied
using this approach.
|
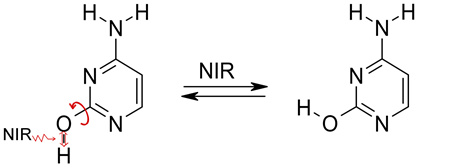
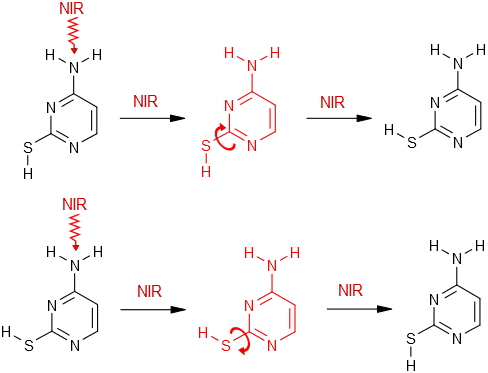
Photoinduced
intramolecular proton transfer
|
PIDA
mechanism: |
|
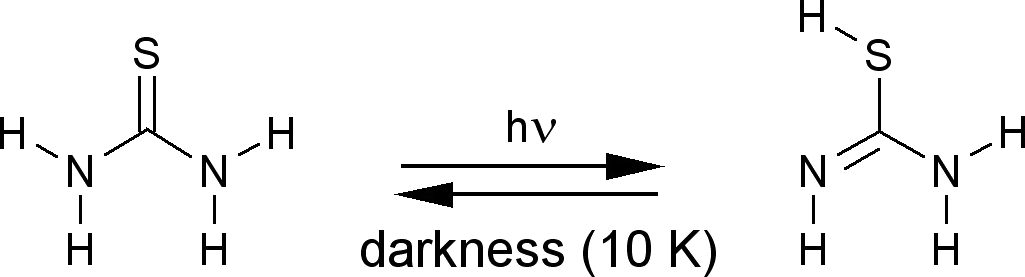
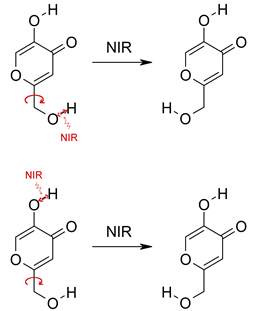
Proton tunneling
(in darkness) following, in some cases, the
phototautomeric reaction
|
 |
Proton tunneling, the spontaneous processes changing the conformational structure |
|
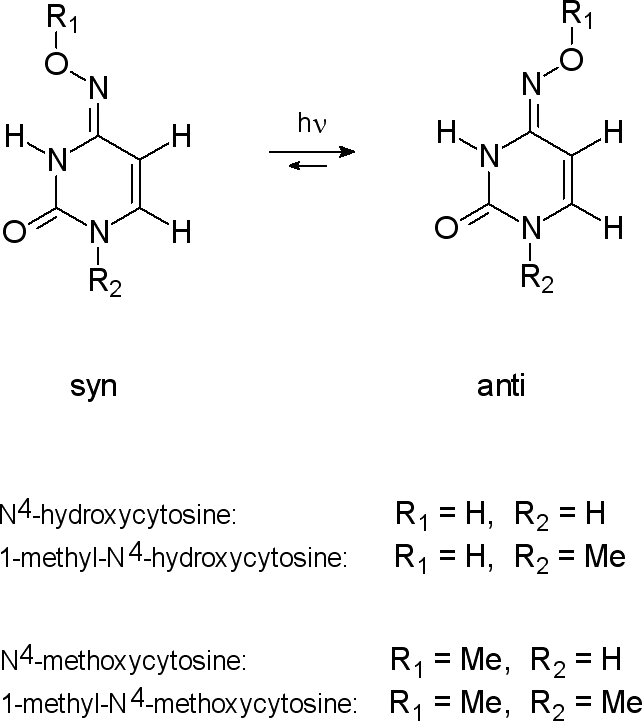
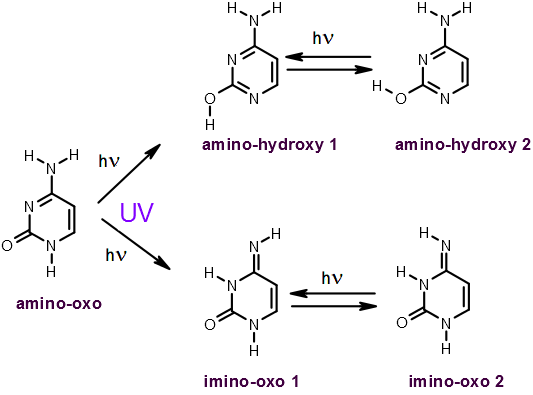
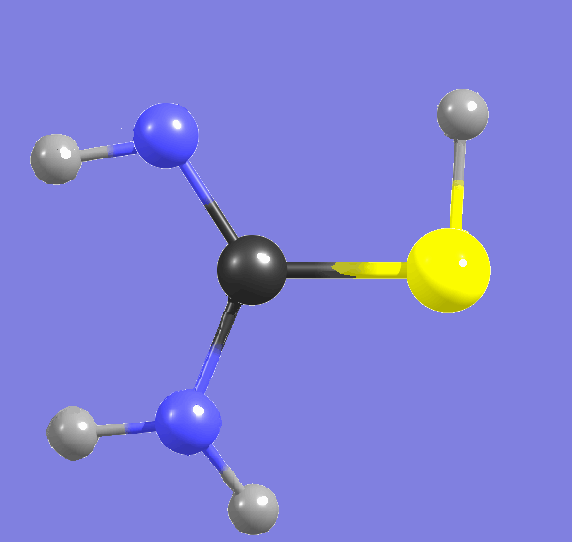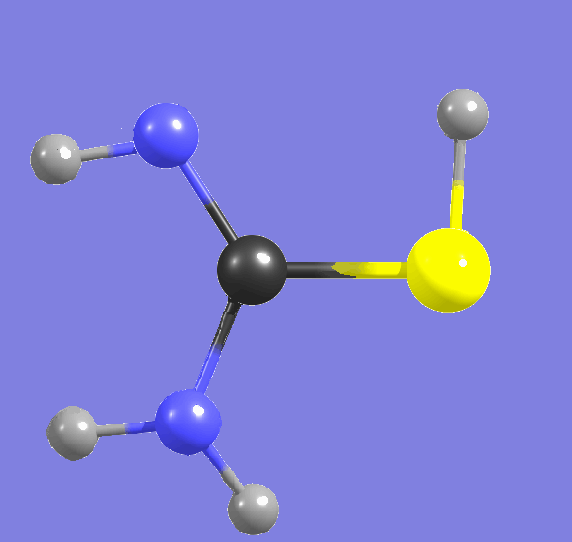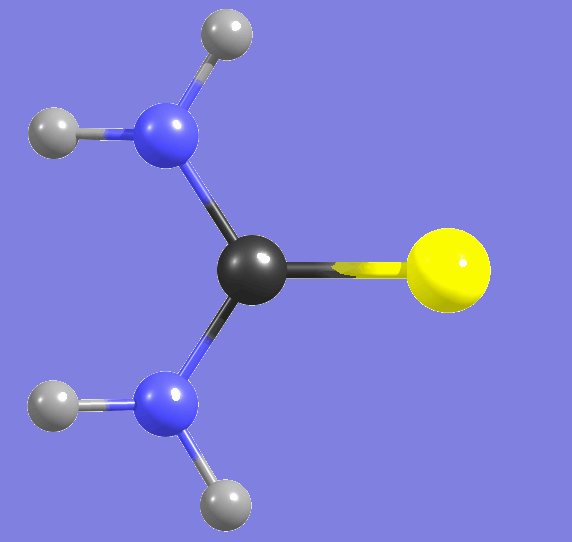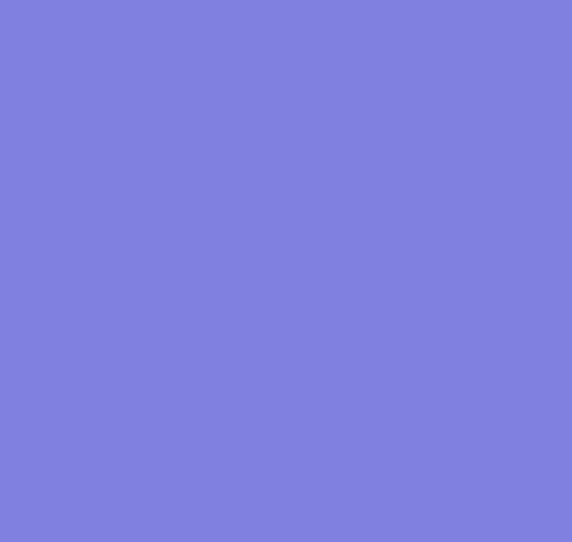
Cytosine,
amino-hydroxy tautomer  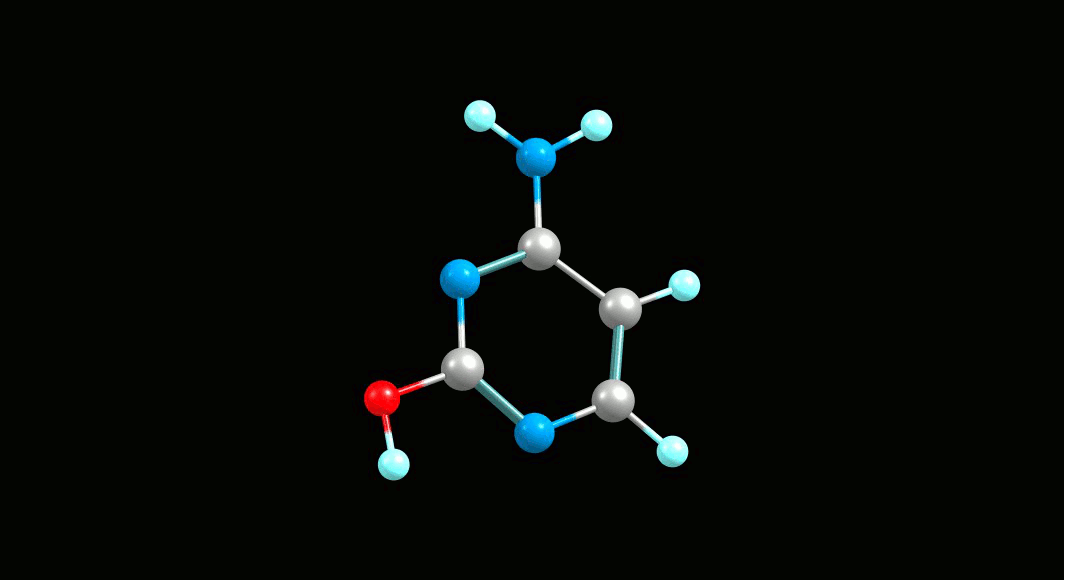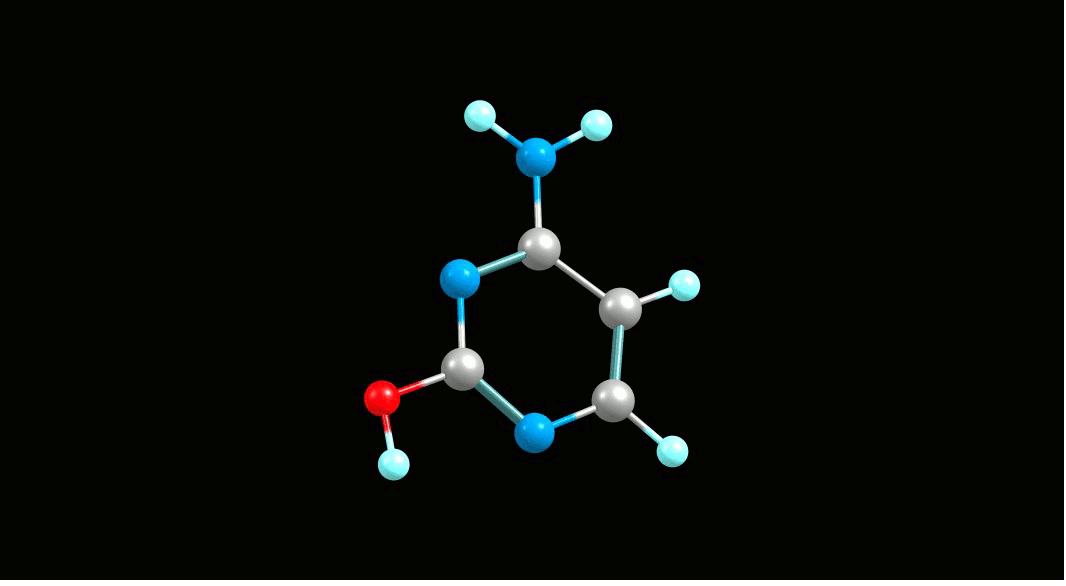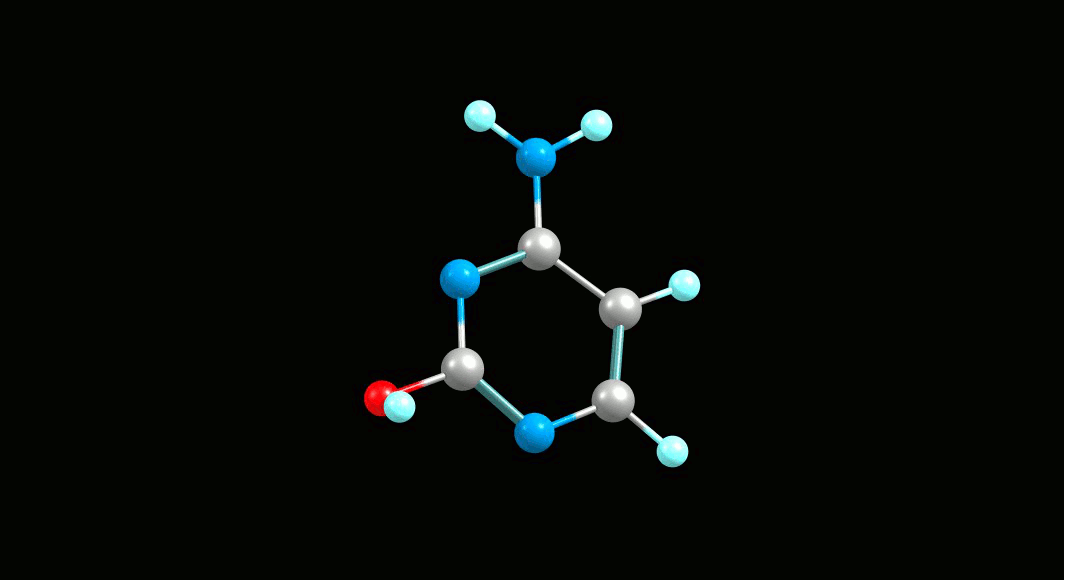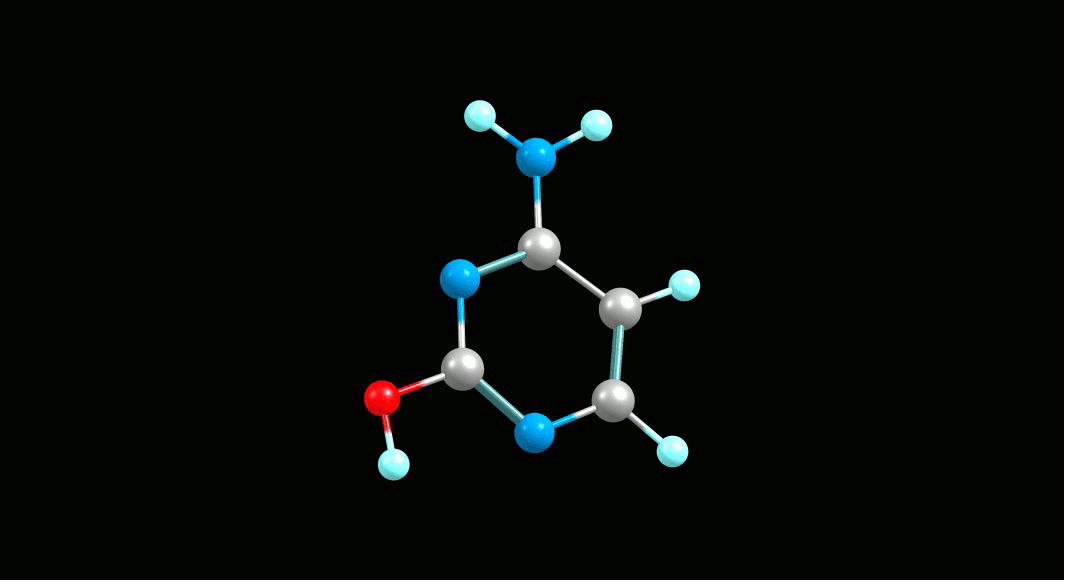 |
|
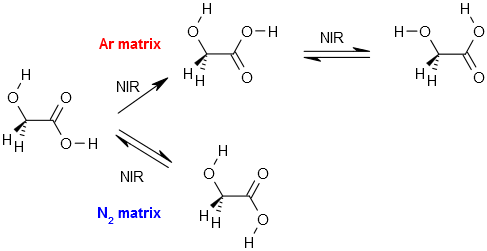
Oxamic Acid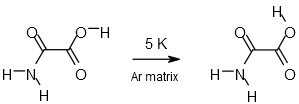 |
2‑Furoic Acid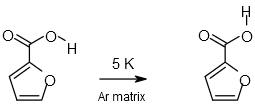 |
HOME