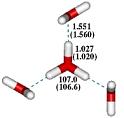
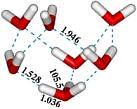
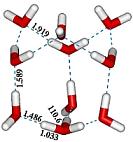
 |
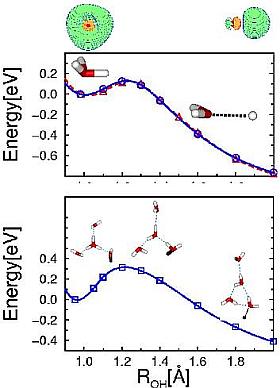 |
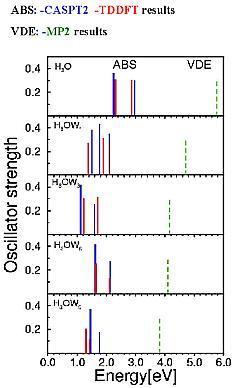
Fig.6 Electronic absorption spectra (ABS)
and vertical detachment energy (VDE)
|
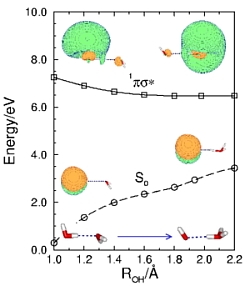
Fig.7 Stability of
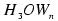 (n=0,3) clusters (n=0,3) clusters
|
Conclusions
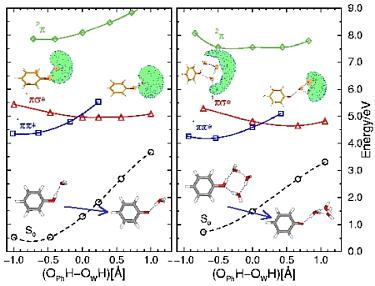
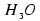 radicals can be formed by a barrierless hydrogen-transfer reaction
in the lowest excited singlet state of water clusters or in the lowest
radicals can be formed by a barrierless hydrogen-transfer reaction
in the lowest excited singlet state of water clusters or in the lowest 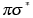 state of an organic chromophore complexed with water.
state of an organic chromophore complexed with water.
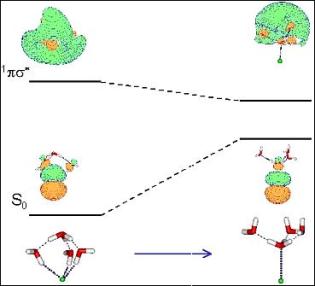
Microsolvation of the 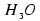 radical leads to a charge-separated complex,
consisting of a hydronium radical and a localized electron cloud, connected by a water network.
radical leads to a charge-separated complex,
consisting of a hydronium radical and a localized electron cloud, connected by a water network.
The vertical excitation energies of the s p electronic transitions of the 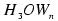 clusters cover roughly the energy range of the absorption spectrum of the hydrated electron.
clusters cover roughly the energy range of the absorption spectrum of the hydrated electron.
Microsolvation with water stabilizes considerably the (unstable) 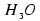 radical, but for n=3 it is still a metastable species which can decay by detaching the hydrogen atom.
radical, but for n=3 it is still a metastable species which can decay by detaching the hydrogen atom.
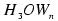 clusters could be the carriers of the characteristic properties of the hydrated electron.
clusters could be the carriers of the characteristic properties of the hydrated electron.
|
References
1. A. L. Sobolewski and W. Domcke, "Photoinduced electron and proton transfer in phenol
and its clusters with water and ammonia",J. Phys. Chem. A 105 (2001) 9275
2. A. L. Sobolewski and W. Domcke, "Hydrated hydronium: a cluster model of the solvated
electron?", Phys. Chem. Chem. Phys., 4 (2002) 4
3. A. L. Sobolewski, W. Domcke, "Photochemistry of 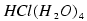 : a cluster model of the
photodetachment of the chloride anion in water", J. Phys. Chem. A, submitted
: a cluster model of the
photodetachment of the chloride anion in water", J. Phys. Chem. A, submitted
|